Japanese Center for the Validation of Alternative Methods
Office : New Testing Method Assessment, Division of Pharmacology,
National Biological Safety Research Center (NBSRC),
National Institute of Health Sciences (NIHS)
About JaCVAM
- Home >
- About JaCVAM >
- Policy and Mission of JaCVAM
Purpose of establishment of JaCVAM
- To promote the 3Rs (Reduction、Refinement、Replacement) in animal experiments for the evaluation of chemical substance safety in Japan.
- To establish guidelines for new alternative experimental methods through international collaboration.
The roles of JaCVAM
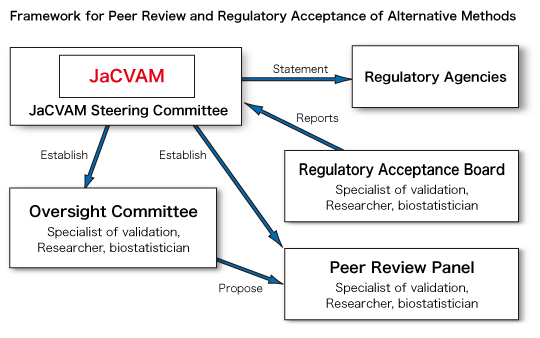
(1) The evaluation of new and revised testing methods and establishment of their guidelines
The evaluation of new and revised testing methods and establishment of guidelines for such methods are processed in the following order: open recruitment, receiving applications, selection for evaluation, preparation of Background Review Document (BRD) by an oversight committee, evaluation by a peer review panel, evaluation by a regulatory acceptance board, and proposal to regulatory bodies.
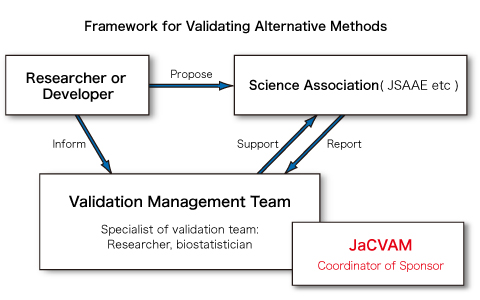
(2) Outsourcing the validation of new and revised testing methods
If a validation result is found to be unsatisfactory in the evaluation process and a further validation is deemed necessary, the director of JaCVAM will outsource the validation to an appropriate scientific society, such as the Japanese Society for Alternatives to Animal Experiments (JSAAE). The director of JaCVAM will ask them to form an ad hoc management team for the validation study of each method and to perform a comprehensive validation process including the preparation of reports. The secretary will support the smooth execution of the validation study. In addition, the oversight committee will collaborate with them to make BRD available for the peer review panel when appropriate.
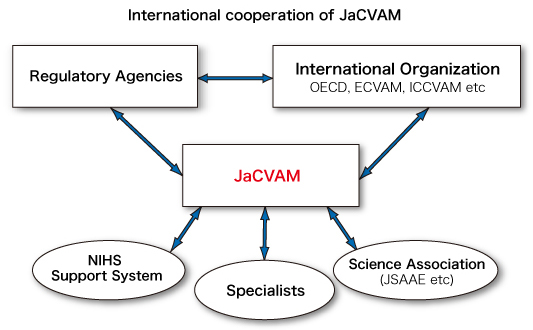
(3) Promotion of the 3Rs and international collaborations
To promote the 3Rs in Japan, the director of JaCVAM collects information about the 3Rs from Japan and abroad and broadcasts this information through scientific societies, web sites, and publications, as well as by to holding symposia as the occasion demands. Additionally, the secretary distributes publications that summarize the JaCVAM’s activities to the relating organizations.
The director of JaCVAM collaborates on promotion of the 3Rs with international organizations for research and associated activities in many areas, including evaluation and validation of new/revised testing methods.